The National Agency for Food and Drugs Administration Control (NAFDAC), has alerted Nigerians on substandard syrups and medicines identified in the WHO regions of the Americas, Eastern Mediterranean, South-East Asia, and Western Pacific.
NAFDAC disclosed this on X ( formerly known as Twitter).
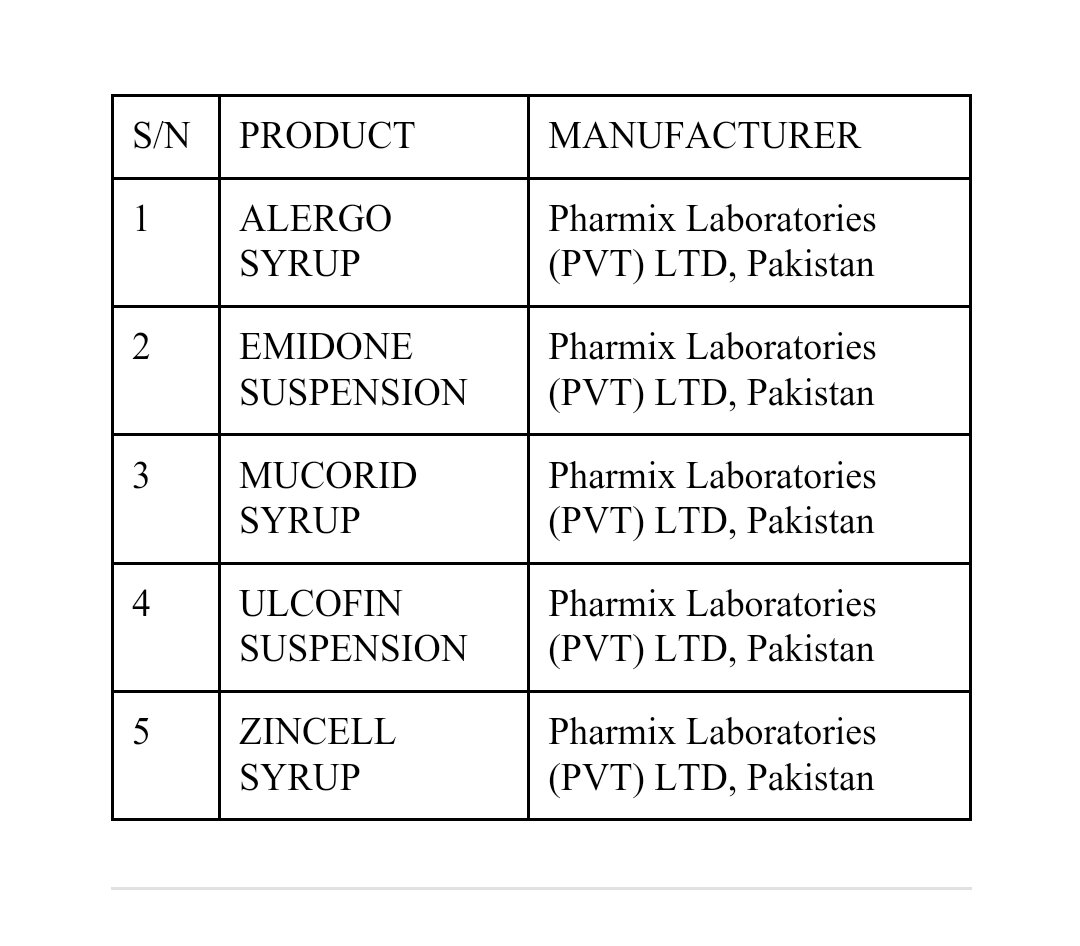
According to NAFDAC, it’s notifying healthcare providers and the public of the WHO medical product alert on five (5) contaminated syrup and suspension medicines initially detected in the Maldives and Pakistan.
“The five products are ALERGO Syrup, EMIDONE Suspension, MUCORID Syrup, ULCOFIN Suspension, and ZINCELL Syrup.
A total of 23 batches of these products are affected. The stated manufacturer of all the affected products is PHARMIX LABORATORIES (PVT.) LTD (Pakistan).
The agency warned that the these substandard products referenced in this alert are unsafe and their use, especially in children, may result in serious injury or death.
It stated that although these products are not registered by NAFDAC, they may have been distributed, through formal and informal markets, to other countries or regions including Nigeria.
NAFDAC implored importers, distributors, retailers and consumers to exercise caution and increase vigilance within the supply chain to avoid the importation, distribution, sale and use of the substandard cough syrups.
NAFDAC directed that all medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.